Global Neurology Clinical Trials Market to Hit USD 11.78 Bn by 2035 | CAGR 6.5%
27 Aug 2025 | Report ID: MI3491 | Industry: Healthcare | Pages: 220 | Forecast Year: 2025-2035
Read more about this report- Global Neurology Clinical Trials Market to Hit USD 11.78 Bn by 2035 | CAGR 6.5%
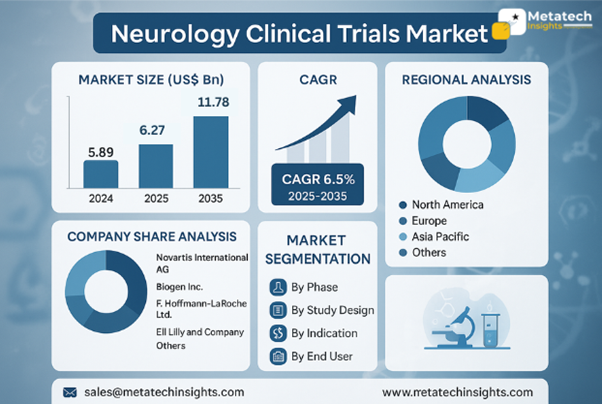
- The Neurology Clinical Trials Market is valued at USD 5.89 billion in 2024.
- The Neurology Clinical Trials Market will achieve USD 11.78 billion by 2035 through a projected 6.5% CAGR from 2025 to 2035.
- The neurology clinical trials market is also recording a considerable growth trend due to the escalating number of neurological disorders, the surging spending in development and research, and heightened awareness of new therapies. Improvement in regulation, technological innovation as far as trial design is concerned, and utilization of digital tools when it comes to patient monitoring are further catalyzing market growth.
- The demand for personalized medicine, or targeted therapies, is offering new possibilities in clinical trial sponsorship and research organizations around the world. Market segmentation is segmented based on phase, study design, indication, and end-user. Depending on the phase, the trials could be between Phase I and Phase IV; however, the most common ones are Phase II and Phase III trials since they are important to test efficacy and safety.
- The study design segments are randomized controlled trials, observational studies, and adaptive trials, which signify the need to have a strong and scientifically sound design. The indications cover Alzheimer's disease, Parkinson's disease, multiple sclerosis, epilepsy, and other neurological diseases, es, withperately needed fields, with Alzheimer's and Parkinson's showing the greatest demands.
- The end-users of the market are pharmaceutical and biotechnology firms, contract research organizations (CROs), hospitals and medical centers, and academic and research institutes. The pharmaceutical and biotech firms make up most of the trials, utilizing clinical trials to determine the usefulness of the new therapeutics, with CROs and academic facilities contributing through their modern expertise, patient sourcing, and data management.
- Increasing development of technology, e.g., AI-based process of selecting patients, remote monitoring, and electronic capture of data, and the growing partnerships between industry and research organizations all support the market. The increased recent interest in patient-centered methods, along with government incentives regarding neurological research, is improving patient enrollment rates and trial efficiency.
- Geographically, the North American market leads the market due to increased R&D spending, the large presence of the major pharmaceutical companies, and the established structure in place to conduct clinical trials. Europe occupies a large proportion owing to regulatory advantage and health spending, whereas the Asia Pacific region is recording the highest growth owing to the rising awareness of neurological conditions and expanding health infrastructure, in addition to cost-efficient clinical trial activities.
- The major forces that contour the neurology clinical trials market are Novartis International AG, Biogen Inc., F. Hoffmann-La Roche Ltd., Eli Lilly and Company, and other international organizations. These corporations are concentrated on strategic trial design, innovative therapy development, patient-centric models, and strategic alliances with research organizations in the bid to enact their market presence and enhance the development of next-generation neurological therapies.
Maximize your value and knowledge with our 5 Reports-in-1 Bundle - over 40% off!
Our analysts are ready to help you immediately.
