Animal Stem Cell Therapy Market By Product Type (Autologous Stem Cell Therapy and Allogeneic Stem Cell Therapy), By Type of Stem Cells (Mesenchymal Stem Cells, Hematopoietic Stem Cells, Neural Stem Cells, and Induced Pluripotent Stem Cells), By Application (Osteoarthritis, Skin regeneration, Neurodegenerative diseases, and Autoimmune diseases), Species (Dogs, Cats and Horses), and End-User (Veterinary Clinics, Research Laboratories and Universities and Academic Institutions ), Global Market Size, Segmental analysis, Regional Overview, Company share analysis, Leading Company Profiles And Market Forecast, 2025 – 2035
Published Date: Sep 2024 | Report ID: MI1067 | 220 Pages
What trends will shape this market in the coming years?
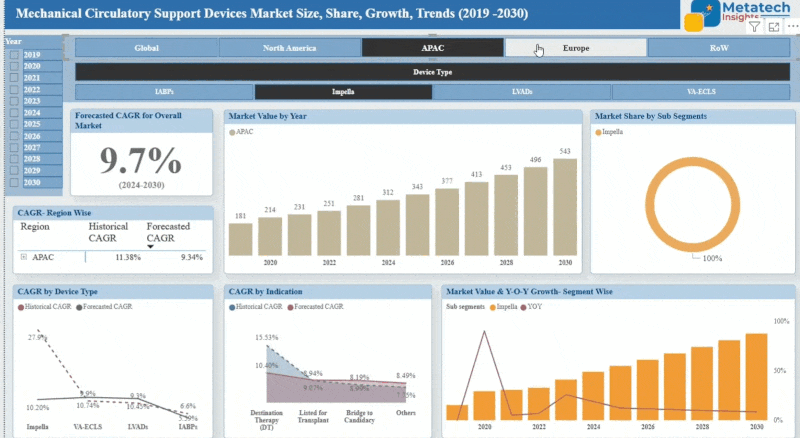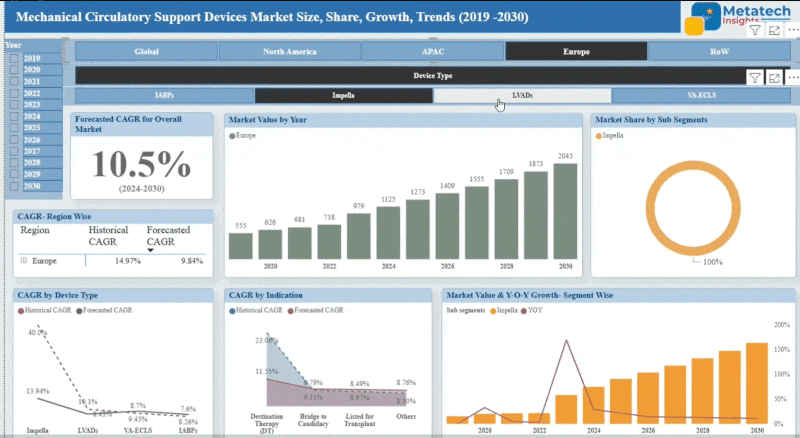
Animal Stem Cell Therapy market accounted for USD 782.4 Million in 2024 and is expected to reach USD 2,144.6 Million by 2035, growing at a CAGR of around 9.6% between 2025 and 2035. Animal stem cell therapy employs stem cells to treat a variety of medical conditions in animals such as arthritis, spinal cord injuries, and dermatological conditions. These stem cells are derived either from adipose tissue or bone marrow and can repair and rebuild damaged tissues leading to better health outcomes. Animal stem cell therapy has become increasingly accepted due to the potential benefits and gains in veterinary medicine. Animal stem cell therapy promises new alternative methods of classical treatments but also faces high costs as well as restraints from regulatory aspects. With pet owners and veterinary surgeons searching for innovative solutions for chronic and degenerative diseases, the Animal stem cell therapy market is growing.
What do industry experts say about the market trends?
“We have provided PRP mechanical and gravity separation devices to the industry for over a decade. I am excited to bring this innovative easy to use and consistent product to the field, one that every veterinarian can use in their practice. PrecisePRP is game-changing for the general practitioner because it makes PRP more accessible than ever before. It takes the guesswork out of PRP therapy so that veterinarians know that the PRP they are injecting has been quality tested for sterility, platelet count, and potency. This means providing a more consistent product every time.”
— Kristi Hauta, Director of Commercial Operations
''We continue to invest heavily in patent protection of our technology and have successfully negotiated royalty-bearing licenses with multiple human and veterinary stem cell companies.”
— Dr. Bob Harman, VetStem CEO
“We are excited to have Dr. Hale join the team. She brings significant experience in product development and regulatory approval. We plan to tap into her vast knowledge and skillset to expand PSC’s clinical trials and product offerings.”
— Dr. Bob Harman PSC founder and CEO
Which segments and geographies does the report analyze?
| Parameter | Details |
|---|---|
| Largest Market | Asia Pacific |
| Fastest Growing Market | North America |
| Base Year | 2024 |
| Market Size in 2024 | USD 782.4 Million |
| CAGR (2025-2035) | 9.6% |
| Forecast Years | 2025-2035 |
| Historical Data | 2018-2024 |
| Market Size in 2035 | USD 2,144.6 Million |
| Countries Covered | U.S., Canada, Mexico, U.K., Germany, France, Italy, Spain, Switzerland, Sweden, Finland, Netherlands, Poland, Russia, China, India, Australia, Japan, South Korea, Singapore, Indonesia, Malaysia, Philippines, Brazil, Argentina, GCC Countries, and South Africa |
| What We Cover | Market growth drivers, restraints, opportunities, Porter’s five forces analysis, PESTLE analysis, value chain analysis, regulatory landscape, pricing analysis by segments and region, company market share analysis, and 10 companies with scope for including additional 15 companies upon request |
| Segments Covered | Product Type, Type of Stem Cells, Application, Species, End-User and Region |
To explore in-depth analysis in this report - Request Sample Report
What are the key drivers and challenges shaping the market?
- Advancements in Veterinary Diagnostics
Advancements in veterinary diagnostics is driving the animal stem cell therapy market growth. Refined diagnostic tools help veterinarians better identify and understand various conditions in animals, leading to personalized stem cell treatments. Improved diagnostics enable better targeting and monitoring of treatments, resulting in an increased adoption rate of stem cell therapies. Technologies such as advanced imaging and genetic testing have expanded the scope of veterinary practices, leading to a greater demand for stem cell therapies. Improved diagnostics are therefore essential for driving innovation and growth in the animal stem cell therapy market.
- Uncertain adverse effects may limit the adoption of stem cell therapies
Adoption of stem cell therapies for animals will be impacted by unpredictable adverse effects. Such risks of adverse effects serve as strong restraints in animal stem cell therapy by fears that the treatments are unsafe and the long-term effects. Adverse effects can include immune reactions, unwanted tissue formation, or process-related complications arising from the procedure of administration of the stem cell. With limited clinical outcomes only reported from longitudinal studies, uncertainty has been introduced and is very likely to hold back veterinarians and pet owners from opting for such therapies. The risks of stem cell treatments are enormous and should, therefore, be mitigated through rigorous testing and monitoring for enhanced credibility of the processes involved.
- Innovative Combination Therapies Drive Growth and New Possibilities in Stem Cell Therapy
Combination therapies offer a promising opportunity within the animal stem cell therapy market. This approach combines stem cell treatments with other therapies, ultimately providing a more comprehensive treatment for animals. It might prove helpful if it is used in combination with regenerative medicine, physical therapy, and gene therapy for the treatment of chronic diseases in animals. It would also show better results about time taken for recovery and recovery outcome. This integrative approach may also expand treatment options and address more diverse conditions, thereby opening the door to stem cell therapy for veterinarians and pet owners.
What are the key market segments in the industry?
Based on the product type, the animal stem cell therapy market has been classified into autologous Stem Cell Therapy and allogeneic Stem Cell Therapy. The largest and most significant segment of the animal stem cell therapy market is autologous stem cell therapy. This is because it has a higher safety profile and is more effective. It primarily uses stem cells extracted from the same animal, which limits the possibility of immune rejection and complications. This approach is favoured, especially due to its reduced possibilities of adverse reactions, so veterinarians are particularly more willing to opt for it.
Based on the type of stem cells, the animal stem cell therapy market has been classified into mesenchymal stem cells, hematopoietic stem cells, neural stem cells, and induced pluripotent stem cells. Mesenchymal stem cells (MSCs) are currently the most predominant type of cells in the treatment market due to their broad applicability and versatility in regenerative medicine. MSCs can differentiate into multiple cell types, including bone, cartilage, and muscle cells, making them suitable for a wide range of therapeutic applications such as treatment for osteoarthritis, spinal cord injuries, and cardiovascular diseases. These stem cells are available in large numbers, can be easily isolated, and support repair in virtually all tissue types. Additionally, increasing clinical evidence confirms the efficacy of MSCs.
Which regions are leading the market, and why?
The Animal Stem Cell Therapy market is experiencing rapid growth in North America. North America's leading position is further strengthened by giant pharmaceutical companies and specialized providers of stem cell therapy. Additionally, the region benefits from a well-established regulatory framework that supports clinical trials and the commercialization of new therapies. All these factors contribute to the high market share held by North America and its rapid growth trajectory.
The Asia-Pacific region is experiencing significant growth in the Animal Stem Cell Therapy Market. This is due to the increased use of advanced veterinary treatments and greater awareness among pet owners and veterinarians in countries like China, Japan, and South Korea. The expanding middle class in these countries is leading to higher spending on pet healthcare, including advanced therapies. Additionally, favorable government policies and investments in biotechnology are strengthening research capabilities and infrastructure within the region. As a result, the Asia-Pacific region is witnessing a remarkable increase in market size and growth rate, establishing itself as a major player in the global animal stem cell therapy landscape.
What does the competitive landscape of the market look like?
Animal stem cell therapy market is highly competitive, with an increasing number of major players and creative strategies. Key operating players in the Animal Stem cell Therapy market are VetStem Biopharma, U.S. Stem Cell, Inc., Medivet Biologics LLC, Cell Therapy Sciences, VetCell Therapeutics, Celavet Inc., Animal Cell Therapies, Inc., Kintaro Cells Power, J-ARM, Magellan Stem Cells, Animal Stem Care, StemBioSys Inc., Pluristem Therapeutics, ZyGEM Corporation and Vetsource. The market dynamics are influenced by regulatory challenges and variations in veterinarians' acceptance of advanced treatments. However, collaborations between biotech firms and veterinary clinics are enhancing access to these treatments. For instance, In May 2023, The US-based Company StemCures announced a substantial $54 million investment to open the largest stem cell production facility in India in Hyderabad. Furthermore, Stempeutics Research has achieved great progress by bringing Stempeucel, the first allogeneic stem cell therapy developed in India, to the global medical community.
Animal Stem Cell Therapy Market, Company Shares Analysis, 2024
To explore in-depth analysis in this report - Request Sample Report
Which recent mergers, acquisitions, or product launches are shaping the industry?
- In April 2024, VetStem, Inc. received FDA clearance for its leucoreduced, allogeneic, pooled, freeze-dried platelet-rich plasma (PRP) product, PrecisePRP Dogs. This product is the first animal cell, tissue, and cell-and-tissue-based product (ACTP) intended for intra-articular use to undergo FDA review and receive this determination. The approval of this off-the-shelf product was aimed at signaling a significant breakthrough in regenerative medicine for veterinarians.
- In 2022, In order to treat knee osteoarthritis (OA), Stempeutics and Alkem Laboratories introduced "StemOne," the first off-the-shelf cell treatment product "made in India." In order to treat knee OA, it is the first allogeneic cell therapy product that has been approved for use in the Indian market.
Report Coverage:
By Product Type
- Autologous Stem Cell Therapy
- Allogeneic Stem Cell Therapy
By Type of Stem Cells
- Mesenchymal Stem Cells
- Hematopoietic Stem Cells
- Neural Stem Cells
- Induced Pluripotent Stem Cells
By Application
- Osteoarthritis
- Skin regeneration
- Autoimmune diseases
- Neurodegenerative diseases
Species
- Dogs
- Cats
- Horses
End-User
- Veterinary Clinics
- Research Laboratories
- Universities and Academic Institutions
By Region
North America
- U.S.
- Canada
Europe
- U.K.
- France
- Germany
- Italy
- Spain
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Singapore
- Rest of Asia Pacific
Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
List of Companies:
- VetStem Biopharma
- U.S. Stem Cell, Inc.
- Medivet Biologics LLC
- Cell Therapy Sciences
- VetCell Therapeutics
- Celavet Inc.
- Animal Cell Therapies, Inc.
- Kintaro Cells Power
- J-ARM
- Magellan Stem Cells
- Animal Stem Care
- StemBioSys Inc.
- Pluristem Therapeutics
- ZyGEM Corporation
- Vetsource
Frequently Asked Questions (FAQs)
Animal Stem Cell Therapy market accounted for USD 782.4 Million in 2024 and is expected to reach USD 2,144.6 Million by 2035, growing at a CAGR of around 9.6% between 2025 and 2035.
The demand for Animal Stem cell Therapy is on the rise due to Combination therapies offering a promising opportunity for market expansion. This approach combines stem cell treatments with other modalities of therapy, ultimately providing a more comprehensive treatment for animals.
Mesenchymal Stem Cells (MSCs) represent the largest and fastest-growing segment. This is due to their versatility, ease of isolation, and ability to differentiate into a variety of cell types, including bone, cartilage, and fat cells. Induced Pluripotent Stem Cells (iPSCs) are also gaining traction due to their potential for personalized therapies and reduced ethical concerns, making them a rapidly growing segment.
The largest region in the Animal Stem Cell Therapy market is North America, driven by advanced veterinary healthcare infrastructure, high pet ownership rates, and increasing adoption of regenerative therapies.
Key operating players in the Animal Stem cell Therapy market are VetStem Biopharma, U.S. Stem Cell, Inc., Medivet Biologics LLC, Cell Therapy Sciences, VetCell Therapeutics, Celavet Inc., Animal Cell Therapies, Inc., Kintaro Cells Power, J-ARM, Magellan Stem Cells, Animal Stem Care, StemBioSys Inc., Pluristem Therapeutics, ZyGEM Corporation and Vetsource
Maximize your value and knowledge with our 5 Reports-in-1 Bundle - over 40% off!
Our analysts are ready to help you immediately.